Trudhesa Difference

Trudhesa™ unites a trusty drug with gentle delivery
Time-tested and long-lasting
The medication in Trudhesa is called DHE or, if you want to get technical, dihydroergotamine mesylate. It has been used by specialists and hospitals for years. DHE is known for working when nothing else will.
The result from a previous study of DHE with nasal spray delivery showed

In 4 previous studies of people using DHE with an older nasal spray device, many had no pain or mild pain at 2 hours.
Study 1
- About 2 out of 3 people had pain relief
Study 2
- About half of people had pain relief
Studies 3 and 4
- 3 out of 10 people had pain relief


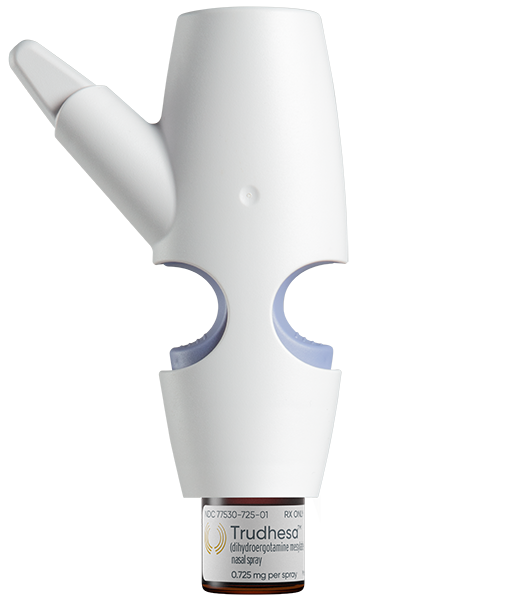
Precision Olfactory Delivery but you can call it POD®
POD is designed to gently deliver medication to the upper nasal space, which is the ideal place in your nose for rapid absorption into your circulation.
See why the upper nasal space is the place to be
View Transcript Close Transcript
TITLE: WHY THE UPPER NASAL SPACE MAY BE THE IDEAL ROUTE
TO RELIEF
VOICE OVER: The upper nasal space is a unique and
underutilized environment for drug delivery. The ciliated olfactory
epithelium in this richly vascularized space makes it ideal for rapid
absorption into the systemic circulation, potentially leading to a faster
response than oral medications. Potential benefits of delivery to this
space also include the reduction of drug-related adverse effects and
potential avoidance of delayed absorption in the GI tract and first-pass
metabolism in the liver.
Traditionally, nasal drug administration has focused on delivery to the
lower nasal space, which is associated with significant drug loss from the
nose with existing delivery systems, which may lead to variability in
absorption. In addition, deep inspiration associated with existing systems
can collapse the nasal valve essentially blocking access to the vascular
rich upper nasal space.
The mucosal layer in the upper nasal space tends to be more permeable and
stable than the lower nasal cavity, permitting consistent, faster and more
extensive absorption of drug into the underlying vasculature with less
mucociliary clearance.
ONSCREEN: WHY THE UPPER NASAL SPACE MAY BE THE IDEAL
ROUTE TO RELIEF
There’s nasal, and then there’s upper nasal
This area of the nose is rich in blood vessels, so medications can be quickly absorbed into your circulation.
It also means that the drug doesn’t need to take the journey through your gastrointestinal (GI) tract before it can start to work.

What relief at the ready could mean for you
And what exploratory results from a 24-week study of the safety showed, where 4515 migraine attacks were treated with Trudhesa.
In some people, Trudhesa was:
- Relief can start in as early as 15 minutes*
- Pain freedom at 2 hours — more than half of people were free from their most bothersome symptom at 2 hours*
- With just 1 dose pain freedom was sustained for 2 whole days*
- 9 out of 10 people who were pain free at 2 hours were still pain free at 48 hours*
- Of all the migraine attacks treated, 85% didn’t require a second dose, and those who got relief with Trudhesa once, were likely to get relief again
- Rates of pain freedom were the same whether people took Trudhesa within 2 hours or several hours into a migraine attack*
*Exploratory efficacy outcomes are based on patient reports and post hoc analyses from a phase 3, open-label safety study of Trudhesa.

Be Direct.
See what people with migraine who took part in the study said:

When people compared Trudhesa to their regular migraine medication, more than half said Trudhesa:
- Worked faster
- Lasted longer
- Was more consistent
- Got them back to normal activities quicker
†Results were from a poststudy questionnaire, which asked 354 people with migraine to rate their experience with Trudhesa against their best usual care. The results were patient-reported and not validated.
Other things you need to know
- Before taking Trudhesa, talk to your healthcare provider (HCP) about all the medications you are taking
- Do not take Trudhesa if you are pregnant, planning to become pregnant, or nursing
- Before taking Trudhesa, talk to your HCP if you have, or think you may have, any cardiovascular (CV) disease risks‡
- Trudhesa was not associated with drowsiness
- Trudhesa can be taken twice within a 24-hour period, if needed, and 3 times over the course of a week
- Generally low levels of nausea were seen with Trudhesa
More Questions? See FAQs >
‡People with migraine were excluded if they had a history of CV events or presented with significant risk factors for CV disease. But people with a history of hypertension, if stable and well controlled on current therapies for >6 months, could enroll if no other risks were present.